Biochemical and structural analysis of Leishmania amazonensis Replication Protein A: a model to study the evolution of telomere-end binding proteins
Telomeres are the structures at the end of eukaryotic chromosomes. They are important for the maintenance of genome homeostasis. Telomeric DNA is associated with specialized proteins, named telomere-end binding proteins (TEBPs), all of which bind to single-stranded DNA via an OB-fold motif. We are interested in the evolution of TEBPs in eukaryotes and particularly in early-divergent eukaryotes as trypanosomatids. Our recent study suggests that the subunit 1 of the Replication Protein A in Leishmania amazonensis (LaRPA1) also acts as a TEBP in trypanosomatids. LaRPA1 is the homologue of the human RPA1 subunit of the heterotrimer RPA, the main single-stranded DNA-binding protein (SSBP) in eukaryotes. The thesis project will conduct an in-depth study of the DNA binding properties and the structure of Leishmania RPA and compare them to the ones of human POT1 (Protection of Telomeres, the human TEBP) and human RPA, in order to understand how telomeres
maintenance mechanisms have evolved in eukaryotes.
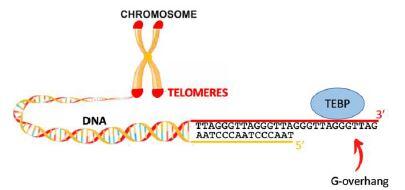
Telomeres are specialized DNA–protein structures that protect the ends of chromosomes in eukaryotes (Figure 1). During evolution, eukaryote telomeres have conserved common features in DNA sequence and nucleoprotein structure. In a variety of eukaryotes, telomeric DNA is composed of a double-strand region, consisting in short tandem G-rich motif repeats on the 5' to 3' oriented strand, and a single-strand extension on the 3’ side, called G-overhang. G-overhangs are specifically associated with telomere-end binding proteins (TEBPs). TEBPs are crucial for telomeres maintenance as they participate to chromosome end protection, telomerase recruitment and regulation of telomere replication. The nature of TEBPs may vary in eukaryotes but all TEBPs contain OB-fold motifs, that are involved in the binding to the telomeric ssDNA or in protein-protein interactions.
Surprisingly, in early-divergent eukaryotes as trypanosomatids, if the telomeric motif is identical to that of the human, no homologues to the classical TEBPs have been found. Our recent studies have confirmed that in the genomes of Leishmania spp there are no homologues to the OB-fold containing telomeric proteins found in other eukaryotes (Fernandes 2020). Importantly, our studies highlight that the protein RPA1 in Leishmania amazonensis (LaRPA1) is a strong candidate for the classical TEBPs at trypanosomatids telomeres. LaRPA1 is the homologue of the RPA1 subunit of the RPA complex constituted of three subunits (RPA1, RPA2 and RPA3) (Figure 2A). RPA is the main eukaryote single-stranded DNA-binding protein and it plays multiple roles in DNA processing pathways genomewide. Genome analysis of Leishmania ssp indicated the presence of the RPA2 subunit but the absence of the RPA3 subunit (Figure 2A).
The thesis project will try to understand this paradox of telomeric maintenance. First, we will determine the telomeres maintenance mechanism of Leishmania amazonensis. As previously done in the SANTE team, we will produce in E. coli and purify the recombinant proteins: LaRPA1 alone or with the LaRPA2 subunit. Then, we will determine the in vitro biochemical and biophysical properties of these proteins by testing their ssDNA binding ability (by Electrophoretic Mobility Shift Assay (EMSA) and Surface Plasmonic Resonance (SPR)), and their capacity to unwind telomeric G-quadruplexes, non-canonical DNA structures adopted by single-stranded telomeric DNA (by fluorescence resonance energy transfer (FRET)) and to drive Liquid-Liquid Phase Separation (LLPS). These data will be compared with data we previously obtained with hRPA and hPOT1 (Lancrey 2018, Chatain 2021). In addition, the structure of LaRPA1, alone or associated with LaRPA2, will be investigated by X-ray crystallography studies, in order to compared these structures to the structures of human RPA and human POT1 (the human TEBP that forms a complex with the telomeric protein TPP1) (Figure (A)). Moreover, we will try to understand how telomeres maintenance mechanisms have evolved in eukaryotes. We will reconstitute chimeric proteins by combining human and Leishmania amazonensis RPA subunits, like LaRPA1/LaRPA2/hRPA3, and by combining RPA and TEBP subunits, like LaRPA1/hTPP1 or hPOT1/LaRPA2 (Figure 2B). We will study whether they form and, if they form, their binding activity and sequence specificity.
This interdisciplinary thesis project will allow to outline a possible scenario of the evolution of an ancestral RPA complex (not sequence-specific and acting genomewide) toward a TEBP complex (specifically interacting with telomeric sequences).
Références
- Chatain J., Hatem G, Delagoutte E, Riou JF, Alberti P, Saintomé C. (2021) Multiple hPOT1-TPP1 cooperatively unfold contiguous telomeric G-quadruplexes proceeding from 3’ toward 5’, a feature due to a 3’-end binding preference and to structuring of telomeric DNA. Nucleic Acids Research. 49(18), 10735-10746.
- Fernandes C., Morea E., dos Santos G., da Silva V., Vieira M., Viviescas M., Chatain J., Vadel A., Saintomé C., Fontes M., Nogueira Cano M. (2020) A multi-approach analysis highlights the relevance of RPA-1 as a Telomere End-Binding Protein (TEBP) in Leishmania amazonensis. BBA - General Subjects 1864(7), 129607.
- Lancrey A., Safa L., Chatain, J., Delagoutte E., Riou J.F., Alberti P., Saintomé C. (2018) The binding efficiency of RPA to telomeric G-strands folded into contiguous G-quadruplexes is independent of the number of G4 units. Biochimie 146, 68-72.
Contacts
- Porteuse du projet : Carole Saintomé - carole.saintome@mnhn.fr, 01 40 79 36 86
Structure et Instabilité des Génomes (Muséum national d’Histoire naturelle - CNRS UMR 7196 - INSERM U1154). Équipe : Structure des acides nucléiques, télomères et évolution (SANTE).
- Doctorante : Marianne Bechara – marianne.bechara@mnhn.fr, 01 40 79 36 84
Appel à projet : AAP 2023